Table of Contents
Toggle
Biocon shares price has climbed 96.5% after the COVID-19 crash to attain an all-time high of Rs. 469 per share. The share price started slipping down and the current market price is traded at Rs. 428.15, accounts for an 8.7% decline from an all-time high.
Indian Biopharmaceutical company released their Q2’2021 results with net profit falling by 22%, even materializing 10% growth in revenue. The fall in growth of profit was the reason for the market to push it back.
Still, the current market price is higher than the value of the company as an Intelligent/Fundamental Investor is a concern. We will be taking you all through a deeper analysis of the company in both Qualitative and fundamental analysis. The quantitative fundamental analysis will be shared in the next article
By the end of this module, you will be clear,
- Why you should invest?
- When you should invest?
- What will be the long-term benefits?
Have a look at the review of Godrej consumer product share price.
History of Biocon Shares Price:
- Back in 1978, the biopharma company was launched by India Women entrepreneur Kiran Mazumdar-Shaw. She made a joint venture with Biocon Biochemicals Limited of Ireland.
- The business kick-off by the manufacture and export of enzymes to the USA and Europe. In 1989 Unilever plc acquired Ireland’s Biocon Biochemicals Limited and merged it with its subsidiary Quest International.
- In this situation, the company received monetary funding from a US company to develop its technologies in the same year. The next year (1990) the company started its in-house research program on solid substrate fermentation technology from pilot to plant level.
- In 1993, the company received ISO 9001 certification from RWTUV, Germany. By 1994, they launched a custom research company (CRC) named Syngene International Pvt. They lead them to a landmark of outsourcing R&D for many pharmaceutical companies inside and outside India.
- In the year 1997, the company made itself ready to launch human healthcare manufacturing facilities. The same year, Biocon became completely Independent, and Unilever sold all the specialty chemicals shareholdings in Quest to Biocon.
- They were completely competent in setting up India’s first Clinical Research Organisation (CRO) in the year for research and development on clinical studies.
- During the year 2001, they got the USFDA approval for the manufacture of Lovastatin. They were the first to receive approval in India for this cholesterol-lowering drug, Lovastatin.
- In the year 2004, it became a public company but was listed in the Stock markets by IPO. The IPO was a successful opening at $1 Billion market share on a listing day.
- Later in the year 2004, the company Sygene launched a new research center “INSUGEN”, a new generation bio human insulin.
- In the year 2009, launched basalog – long-acting basal insulin for both Type 1 & Type 2 insulins.
- During the year 2012, Biocon launched the positive result of a global phase 3 on recombinant human insulin.
- USFDA approved the Biologics License Application (BLA) of Biocon and Mylan on Trastuzumab – An anti-cancer drug.
- In 2019, they launched Trastuzumab in the market for the benefit of patients in India, before which they launched Insulin Glargine, Bevacizumab, and Itolizumab.
Have a look at our review of Discounted Cash Flow Analysis
Business Overview – BIOCON Shares Price:
An Indian Multinational biopharmaceutical company that has changed many patients living in the last 4 decades. They have a presence in over 120 countries globally with their expertise in the areas like,
- Oncology
- Diabetes
- Auto-Immune Disease.
The important driver of the company is to provide quality and high-demand medicines at an affordable price to patients all over the globe. They completely focus on four segments of the pharmaceutical business,
- Generics
- Biologics
- Research Services
- Novel Biologics
Generics:
- Touched the landmark of 2000 Cr annual revenue in FY2019-20.
- Entered into the Chinese market by 3 generic molecules and their licenses.
- Filed new drug application for various APIs
- To enhance production on a fermentation-based manufacturing site in Andhra Pradesh. This will supply demand for the domestic API market.
Biologics:
- The biologics, generally known as Biosimilars have touched almost 2.1 million patients.
- Initiated value unlocking of Biocon Biologics through private equity investment for a minority stake, indicating an equity valuation of USD 3 billion.
- Expanded various biologics globally,
-
- Pegfilgrastim – Australia, Canada.
- Trastuzumab – USA, Australia, Canada.
- Insulin Glargine – Australia.
-
- Got regulatory clearance from the USA and UK for stationing a manufacturing site for Insulin Glargine in Malaysia.
- Integrated R&D facility for 60000 sq. ft in Chennai, Tamil Nadu. This is one of the world-class integrated R&D centers available in India.
- Increased production of drug substances and drug products for key biosimilars through brownfield and greenfield projects.
Research Services:
- Inaugurated new facilities for research services in Hyderabad and Bengaluru.
- The API manufacturing site has been newly launched in Mangalore.
- Extended biologics discovery and preclinical research capabilities in CAR-T therapy, an innovative cell-based approach to treating cancer.
- Received Good Laboratory Practice (GLP) certification for a viral testing facility from the NGCMA, making it India’s first and only GLP-certified viral clearance study service provider.
- Repurposed a high-end laboratory to conduct RT-PCR tests for COVID-19.
- Partnered with Pune-based Mylab to supply reagents for use in its indigenously developed COVID-19 testing kits.
Novel Molecules:
- Started global clinical trials for our first-in-class oral insulin molecule, Insulin Tregopil, in Type 1 diabetes.
- Clinical trials initiated by partner Equillium to study our novel anti-CD6 molecule, Itolizumab, in Lupus Nephritis and severe Asthma.
- Started a clinical trial in India to study Itolizumab in treating moderate to severe patients with COVID-19 complications.
Business Revenue – Biocon Shares Price:
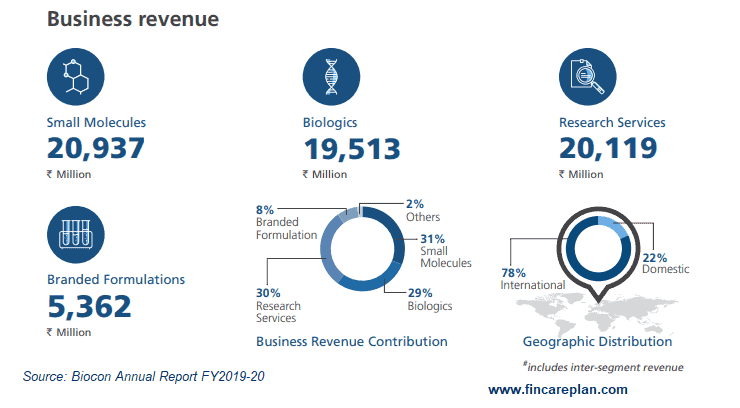
The above image is the snap of business revenue for the financial year 2019-20.
The consolidated revenue of all segments together was Rs. 6528.6 Cr with a growth of 15% over the last financial year. In which the split is as,
- Generics – 31%
- Biologics – 29%
- Research Services – 30%
- Novel Molecules – 8%
- Others – 2%
When we considered the demographics in the revenue split. 22% of revenue comes from domestic business and 78% is dominated by global business.
The company has invested almost 10% of its total revenue in research and development.
Key Strategy Priorities:
- Building a robust portfolio
-
- Forward integration based on strength in APIs.
- Develop Complex Formulations.
- Current API Portfolios
- Gliptins.
- Peptides
- Anti-Obesity
- Immunosuppressants
- Statins
- Fungins
- Oncology
- Multiple Sclerosis
-
- Sustaining the base business.
- Expanding manufacturing base.
- Cost Improvement Initiatives.
- Expanding Commercial footprint
- Strengthening Quality Systems
- people Development.
Pipelines of Products:
- Insulin Tregopil – first-in-class oral prandial insulin molecule for post-prandial glycaemic control
- FmAb2 – Biocon’s immuno-oncology program focusing on the development of novel bi-functional fusion antibodies
- Itolizumab – ALZUMAb™ (Itolizumab), our novel biologic, launched in India for the treatment of chronic plaque psoriasis
Hereby, we conclude the qualitative fundamental analysis of the company. In the next article, we will be taking through the quantitative fundamental analysis.
Happy Learning and Investing!!